Chemicals are Everywhere

As an organization focused on science, we talk a lot about chemicals. When most people think about chemicals, they assume we’re talking about man-made chemicals, synthetic substances produced by scientists. But in reality, everything—plants, animals, rocks, and even people—is made of chemicals.
Chemistry doesn’t just happen in a lab; chemical reactions are happening constantly in nature.
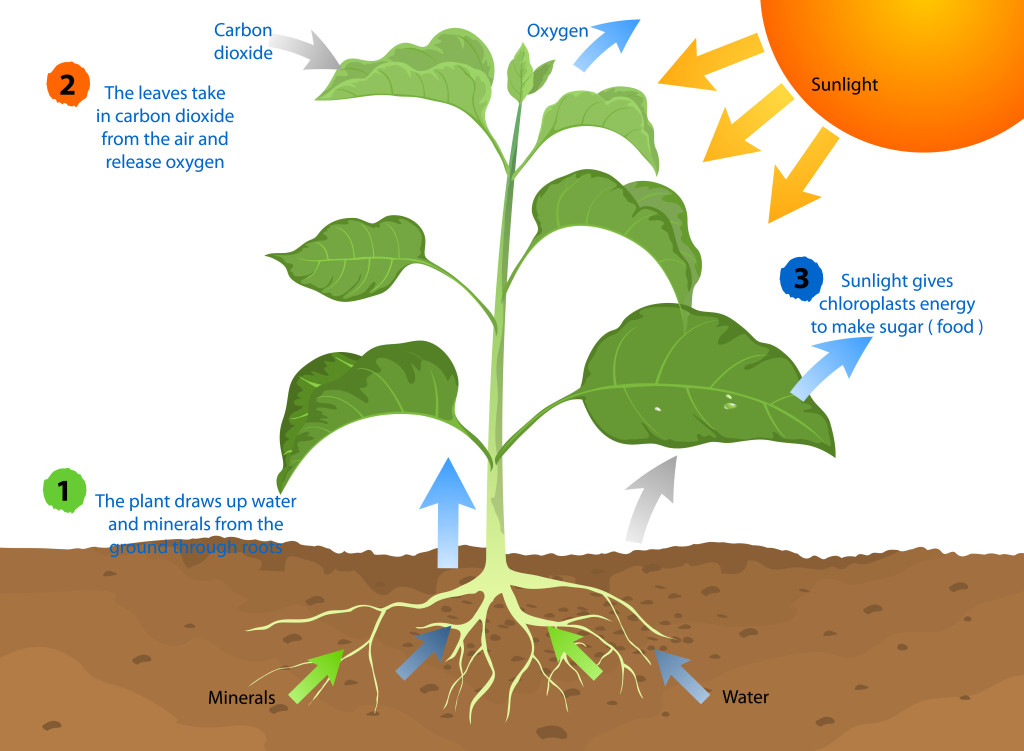
Most of us remember photosynthesis from grade school—it’s how plans convert carbon dioxide into food into oxygen. This chemical reaction can be broken down into the formula:
6 CO2 + 6 H2O + light → C6H12O6+ 6 O2
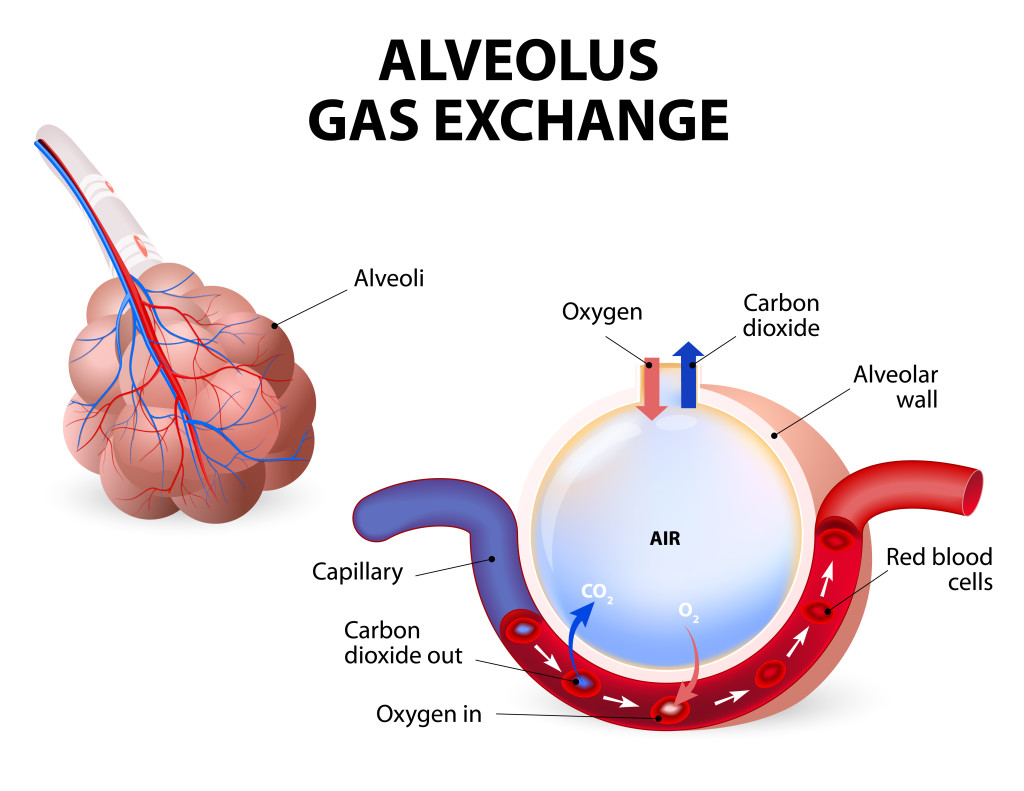
The opposite of this equation is “aerobic cellular respiration,” the process by which living organisms use oxygen to break down food, in the form of glucose (sugar) molecules, into energy. The byproducts of this process are carbon dioxide and water. Here’s the equation:
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy (36 ATPs)
When your favorite cast iron skillet starts to develop red flakes, it’s undergone a chemical reaction: rusting. In what’s known as an “oxidation reaction,” iron develops rust when exposed to water and oxygen. The equation for rusting of iron is:
Fe + O2 + H2O → Fe2O3. XH2O
These are just three examples of the millions of chemical reactions occurring every day. The term “chemical” shouldn’t be used to signify something is synthetic or worse, dangerous. Chemicals are everywhere and without them, life as we know it wouldn’t exist.