FDA Recalls Kim Kardashian’s Latest Instagram Selfie

Celebrity endorsements are nothing new—such marketing ploys go all the way back to the 1700s! Now, these product plugs are everywhere, from traditional media magazine ads and commercials to posts on celebrity social media accounts. But this quest to capitalize on celebrities’ vast social media followings may be leaving out important health information for consumers.
Kim Kardashian, no stranger to selfies, posted a photo to her 42 million Instagram followers showing her holding the morning sickness drug, Diclegis. Kardashian raved to her followers:
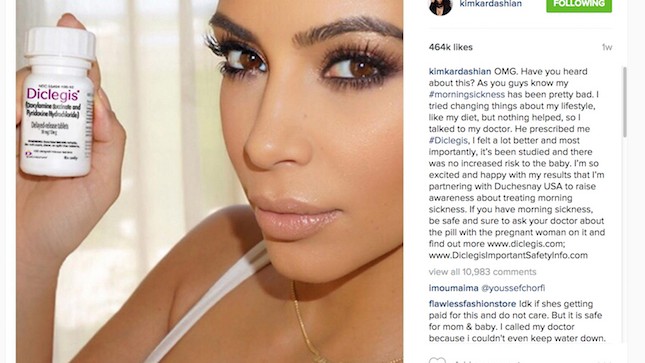
The U.S. Food and Drug Administration (FDA) has strict rules for pharmaceutical ads—each advertisement must give a list of possible drug side effects. Kim’s endorsement of the drug didn’t, and the FDA warned the drug’s manufacturer (which paid Kim for the post) that it could face consequences for Kim’s “misleading” post.
We’ve covered a number of celebrities who have used their massive following to give health advice—often dangerous, scientifically unproven advice (we’re looking at you Suzanne Somers and Gwyneth Paltrow). Unfortunately, FDA doesn’t have the authority to step in and tell Somers’ fans that their high blood pressure is unlikely caused by “an overload of accumulated lead in your bones.” Instead of listening to celebrities for pharmaceutical and health recommendations, talk to your doctor. He or she might not look as attractive holding up a pill bottle, but they’ll be able to tell you whether a drug or treatment is safe and appropriate for you.





